Researchers from Johns Hopkins and Cornell University have discovered that coating zinc-ion batteries’ electrodes with a special polymer extends their life by preventing damage during rapid charging—a crucial advance for these promising energy-storage devices used in everyday electronics and power grids. The team’s results appear in ACS Applied Materials and Interfaces.

Regina García-Méndez
“When these batteries charge too quickly, zinc metal filaments grow inside of them from anode to cathode. When these filaments reach the other electrode, they short-circuit the battery, preventing the battery from charging and rendering it useless,” said team member Regina García-Méndez, assistant professor of materials science and engineering at Johns Hopkins Whiting School of Engineering and core faculty member of its Ralph O’Connor Sustainable Energy Institute (ROSEI).
When conventional zinc-ion batteries are rapidly charged, the ions inside them travel quickly between the anode and cathode—the negative and positive electrodes of the battery—resulting in the formation of slivers of zinc that extend between the electrodes and interfere with the batteries’ performance.
“The metallic strands destroy the inside of the battery and disrupt its ability to hold electricity,” says García-Méndez. “To solve this, we found that adding a small concentration of the polymer to the battery forms a thin protective layer on the electrode’s surface, extending battery life and enabling fast charging rates.”
The team set out to determine whether a specific polymer, polyethylene glycol (PEG), could prevent the zinc ions from forming zinc metal filaments. To test their hypothesis, they experimented with different amounts and sizes of polymer molecules. They checked their results in two types of batteries: small coin-shaped ones and larger pouch-shaped ones, both using water-based electrolytes. To make the electrolyte, they dissolved zinc salt in purified water and then mixed in a polymer called PEG. Using advanced tools like scanning electron microscopy (SEM) to look at the surface details of the zinc electrodes and X-ray photoelectron spectroscopy (XPS) to study their chemistry at the surface, they found that the polymer performed two functions at once within the battery.
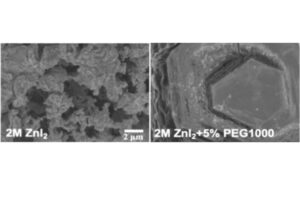
Zinc-ions before adding the polymer, PEG, on the left. When the Polymer is added on the right, the ions rearrange into a uniform structure and stabilize within the battery.
“PEG covered the electrodes with a thin layer which allowed us to prevent a damaging reaction on the anode side of the separator, and the zinc ion rearranged into a uniform structure that prevented filaments from forming,” says García-Méndez. “The polymer protects the battery while simultaneously preventing the metal strands from forming – allowing these batteries to charge faster without fizzling out.”
The team will test this method with larger batteries, aiming to achieve the same results.
“We want to apply the polymer to a larger format with the same conditions and see if it translates. We need to see if this method works when we scale up the materials so that we can apply PEG in everyday zinc-ion batteries,” García-Méndez says.
This study was completed with researchers from Cornell University: Arpita Sharma, Shuo Jin, Donald L. Koch, and Lynden A. Archer of the Robert Frederick Smith School of Chemical and Biomolecular Engineering; Shifeng Hong and Yue Deng of the Department of Materials Science and Engineering; and Ankush Mukherjee of the Sibley School of Mechanical and Aerospace Engineering. The project was supported by the Department of Energy Basic Sciences Energy Program and by the Advanced Research Projects Agency-Energy (ARPA-E).
This story was written by Conner Allen, and originally appeared on the JHU Department of Materials Science and Engineering website.
